>> Avoid Residual Paralysis <<
- Residual neuromuscular block can significantly extend recovery, increasing the risk of serious clinical complications that can lead to increased hospital costs.
- Quantitative TOF monitoring is currently the only method to exclude accurately and consistently RNMB.
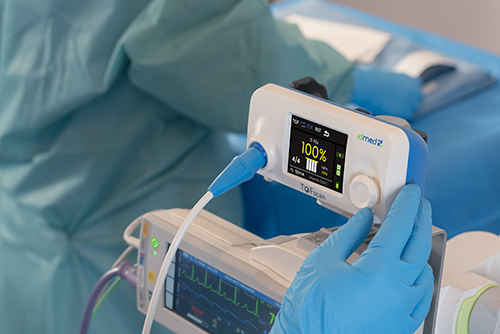
>> Recommendations Worldwide <<
Many societies and their national association of anesthetists around the world recommend NMT quantitative monitoring
in their national guidelines for the management of neuromuscular blockade.
>> Our solution to prevent residual paralysis <<
Enjoy unique clinical benefits of the ToFscan, an easy-to-use 3D-AMG TOF monitor and used worldwide in thousands of operating theaters and intensive care units for almost 10 years

-
Precise and objective measures
-
Three-dimensional accelerometer (3D-AMG)
-
ATP automatic mode (TOF/PTC)
-
No calibration required
>> 3D-AMG, is not AMG <<
The new 3D-AMG and its splint overcome weaknesses of widely accepted gold standard AMG
ToFscan can be used on patients whether having received NMBAs, or not.
The preload on the thumb by the splint ensures consistent and accurate measurements.
>> ToFscan, trusted and proven <<
3D-AMG is clinically equivalent to EMG and AMG

EMG
“Tetragraph (EMG) and ToFscan (3D-AMG) provide INTERCHANGEABLE quantitative measurements once the TOF ratio has returned to a value of 0,90 or greater.”*
AMG
“GOOD AGREEMENT between the TOF-Watch SX (AMG) with calibration and preload application and the uncalibrated ToFscan (3D-AMG) was observed throughout all stages of neuromuscular recovery.**
*Comparison of the Tetragraph (EMG) and ToFscan (3D-AMG) for monitoring recovery from neuromuscular blockade in the Post Anesthesia Care Unit (PACU). DOI: 10.1016/j.jclinane.2021.110234
**Comparison of the TOFscan (3D-AMG) and the TOF-Watch SX (AMG) during Recovery of Neuromuscular Function. DOI: 10.1097/ALN.0000000000002400
>> The ideal NMT monitor for a daily practice <<
Enjoy unique clinical benefits of the ToFscan, an easy-to-use 3D-AMG TOF monitor
RAPID SETUP.
No calibration is required.
COST-EFFICIENT.
Reusable sensors available and no proprietary consumables are required.
EASY-TO-USE.
Continuous and safe monitoring with the « auto pilot » ATP mode.
RELIABLE.
Reinforced immunity against electrosurgical unit interferences.
VERSATILE.
3D-AMG foot sensor available when hands are tucked at patient’s sides.
FOR EVERY PATIENT.
Reusable or single-use 3D-AMG sensors available for different stimulation sites.
>> ToFscan, the TOF monitor for every patient <<
The most comprehensive range of dedicated sensors
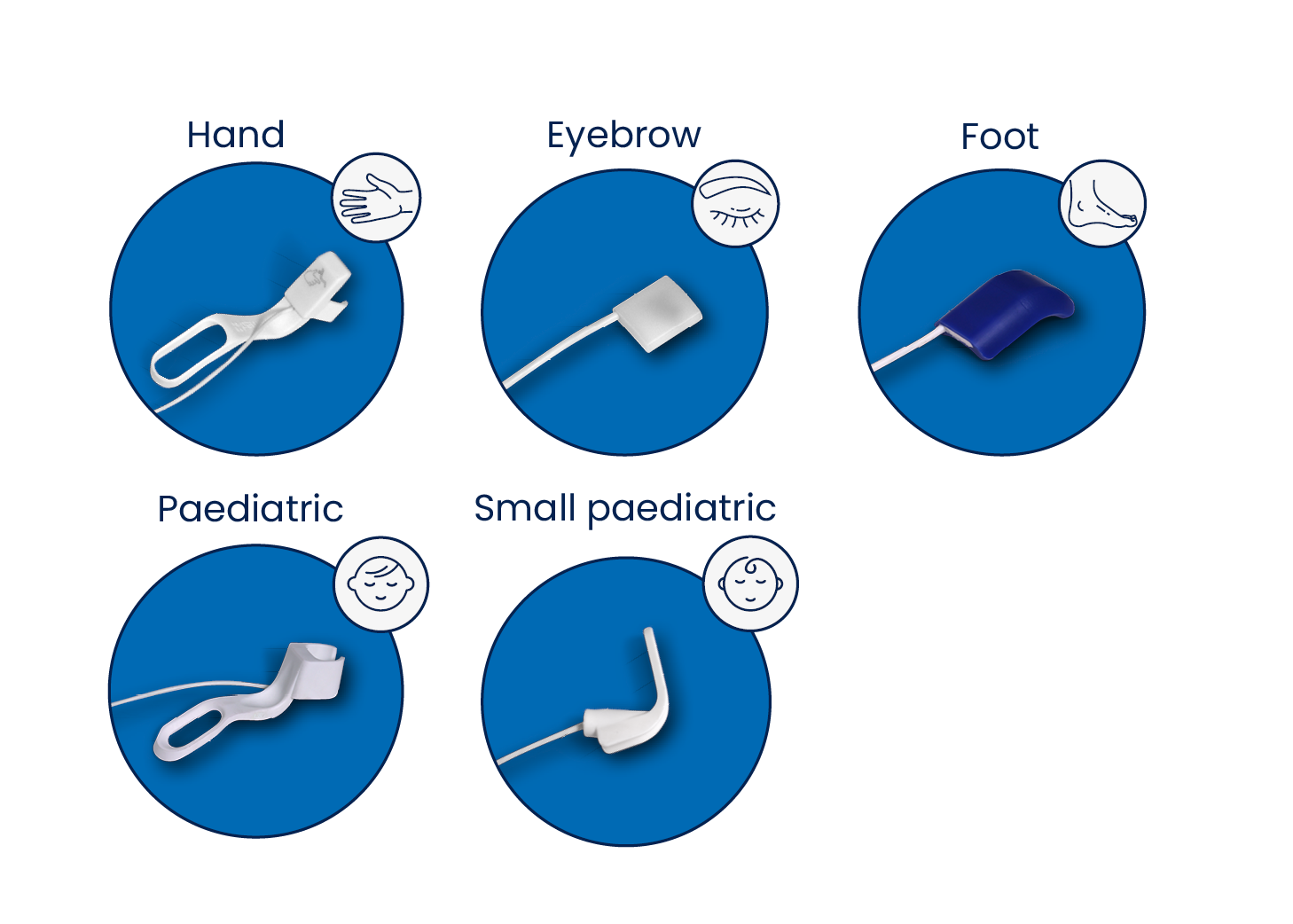
The ToFscan has a range of sensors that answers to all the different medical practices in terms of Neuromuscular monitoring and recovery.
All ToFscan sensors use three-dimensional accelerometry technology. The 3D-AMG technology ensures reliable and accurate measurements and eliminates the need for any calibration process.
>> The most cost-effective TOF monitoring technology available <<
TOFSCAN (3D-AMG) total cost of care is 8 times lower than EMG monitoring
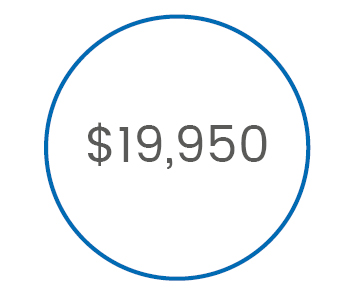
is the annual cost
of implementing
3D-AMG monitoring*
>> 3D-AMG is the less expensive method
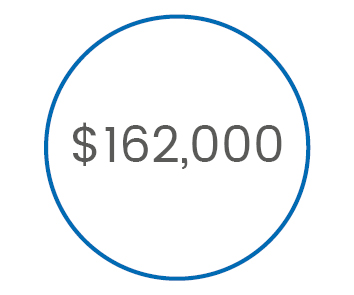
is the annual cost
of implementing
EMG monitoring*
>> EMG costs x8
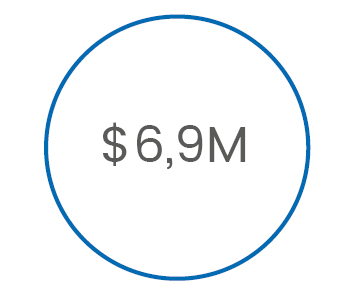
is the annual cost
of post operative
complications**
>> Complications cost x346
Universal quantitative TOF monitoring would result in a
NET COST SAVING in regard to the reduction of post-operative pulmonary complications.
*Depreciated over 5-year usable life span. Include sensors, disposables, and 30 monitors. The same calculation method has been used for EMG and 3D-AMG.
**Lori-Ann Edwards, et al. Perioperative Care and Operating Room Management, ISSN: 2405-6030, Vol: 24, Page: 100184. 2021
>> Excellent in the ICU <<
The ToFscan NMT Monitor fits perfectly in the ICU
Individualised monitoring of your patients
• Optimisation of NMBDs dosage
• Diagnosis of prolonged neuromuscular blockade
• Monitoring of spontaneous recovery
For a smooth practice in intensive care
• For safe endotracheal intubation
• Ensure ventilator-patient synchrony
• To maintain the desired level of block
Integrates into the ICU workflow
• No calibration required
• Rechargeable battery
• Electronic data transfer
• Quick decontamination
Videos
Technical data

STIMULATION
- TOF (Train Of Four)
- Automatic TOF
- ATP (Automatic TOF-PTC)
- PTC
- DBS (3.3, 3.2)
- Single Twitch (0,1 ; 1 Hz)
- Tetanus 50 Hz

MEASUREMENTS
(3D accelerometry)
- TOF mode : T4/T1
- TOF mode : T4/Tref
- TOF mode : count
- PTC mode : count
- DBS mode : count

ERGONOMICS
- Three-dimensional accelerometer sensor
- Adjustable stimulation current (20-60 mA)
- Automatic switch-off
- Battery and mains operated
- Audio message (can be switched off)
- Fixing clamp for bracket
- No calibration required

STANDARDS AND SAFETY
- EN 60601-1 (Medical Electrical Equipment)
- EN 60601-1-2 (EMC)
- 2A CE Class (CE 0459)
- FDA Approved 510k
REFERENCES
*Depreciated over 5-year usable life span. Include sensors, disposables, and 30 monitors. The same calculation method has been used for EMG and 3D-AMG.
- Comparison of the Tetragraph (EMG) and ToFscan (3D-AMG) for monitoring recovery from neuromuscular blockade in the Post Anesthesia Care Unit (PACU). DOI: 10.1016/j.jclinane.2021.110234
- Comparison of the TOFscan (3D-AMG) and the TOF-Watch SX (AMG) during Recovery of Neuromuscular Function. DOI: 10.1097/ALN.0000000000002400
- Lori-Ann Edwards, et al. Perioperative Care and Operating Room Management, ISSN: 2405-6030, Vol: 24, Page: 100184. 2021
- Multicenter Study of the Incidence and Severity of Residual Neuromuscular Blockade DOI: 10.1213/ANE.0000000000000757
- https://sfar.org/utilisation-des-curares-en-reanimation/
- Neuromuscular monitoring and neuromuscular blocking agent shortages when treating critically ill COVID-19 patients: a multicentre retrospective analysis DOI: 10.1016/j.bja.2021.04.028.
- Technologies to Optimize the Care of Severe COVID-19 Patients for Health Care Providers Challenged by Limited Resources DOI: 10.1213/ANE.0000000000004985
- How to reduce cisatracurium consumption in ARDS patients: the TOF-ARDS study DOI: 10.1186/s13613-017-0305-2
- Neuromuscular blockade management in patients with COVID-19 DOI: https://doi.org/10.4097/kja.21106
6A comparison of two depths of prolonged neuromuscular blockade induced by cisatracurium in mechanically ventilated critically ill patients DOI: 10.1007/s00134-002-1508-y - Current Use of Neuromuscular Blocking Agents in Intensive Care Units DOI: 10.5152/TJAR.2019.33269
8Neuromuscular Blockade in the 21st Century Management of the Critically Ill Patient DOI: 10.1016/j.chest.2016.10.040 - Considerations in Neuromuscular Blockade in the ICU: A Case Report and Review of the Literature DOI: https://doi.org/10.1155/2020/8780979
Eco-friendly company

We are an
eco-friendly company

Open ended choice of
disposable or reusable material

Cost
control

Short production
cycle
IDMED WORLDWIDE
Our product range market availability differs for each country. Please contact us to know which product is available in your country.
Our partners and distributors are at your service to meet your needs and the needs of your patients.
![]() Distributeurs implantés
Distributeurs implantés
*Distributors located
![]() Distributeurs en cours d’implantation
Distributeurs en cours d’implantation
*Distributors being implemented

CONTACT US :






 CONTACT
CONTACT











